NOGGO-OV53 / N-PLUS
The world’s first study with the aim of reducing the number of chemotherapy cycles and thus the duration of chemotherapy in patients who have undergone tumor-free surgery. N-PLUS is a study for HRD-positive patientsin primary therapy with advanced ovarian cancer.
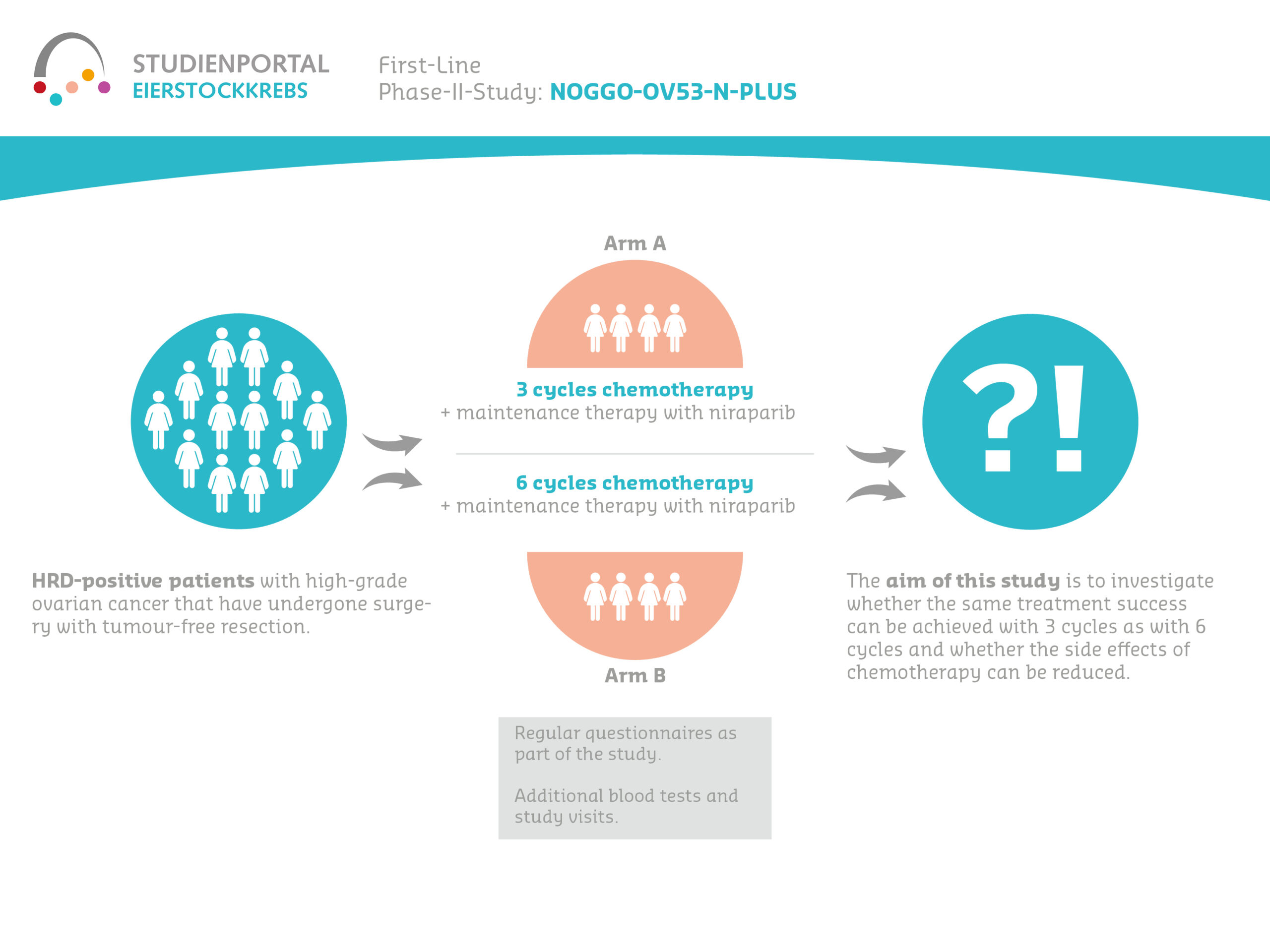
N-PLUS is a randomized (random assignment to treatment arms), non-blinded (all parties know the treatment arm) phase II study of the non-inferiority of niraparib after 3 vs. 6 cycles of platinum-based chemotherapy in tumor-free operated patients with advanced, high-grade HRD positive ovarian cancer in first-line therapy.
What is being investigated in this study?
Until now, it has been standard practice for women with a cancer diagnosis to be treated with 6 cycles of chemotherapy. Chemotherapy is usually associated with side effects that can be severe and can even lead to patients discontinuing treatment. There is evidence from past studies that fewer cycles of chemotherapy followed by treatment with a PARP inhibitor such as niraparib are no less effective than treatment with more cycles – but the chance of having fewer side effects is more likely.
What is the aim of this study?
The aim of this study is to show that the efficacy and safety of three cycles of chemotherapy (carboplatin/paclitaxel) followed by maintenance therapy with niraparib in patients with high-grade ovarian cancer who have undergone macroscopic tumor-free surgery is not inferior to the effect of 6 cycles of chemotherapy plus subsequent maintenance therapy with niraparib. The question is therefore whether the same treatment success can be achieved with 3 cycles as with 6 cycles and whether the side effects caused by the chemotherapy can be reduced.
What is the study procedure?
Patients are randomly assigned (1:1) to one of the two treatment arms, the probability of receiving 3 cycles or 6 cycles of chemotherapy is the same (50:50). Patients complete two 15-minute questionnaires as part of this study, and there are also additional blood tests and study visits. After chemotherapy, niraparib is administered until the cancer returns or for a maximum of 3 years. The tablets are taken daily.
Are there risks?
You will be informed about possible risks and side effects associated with participation during an information session.
Participation Requirements:
This study is open to women aged 18 years and older with:
- HRD-positive, high-grade, advanced ovarian, tubal or primary peritoneal carcinoma
- Stage III or IV
- Complete surgical resection
In addition, there are other criteria that must be met in order to participate in the study. Interested patients should speak to the investigators at a study center, who can check whether this study is suitable for them.
Where can I particapte?
Participating study centres are located in: Austria, Belgium, Czechia, Germany, Italy and Spain.